Abstract
Introduction: Copanlisib plus rituximab (C+R) was recently shown to be superior to placebo plus rituximab (P+R) in prolonging progression-free survival in patients with relapsed indolent non-Hodgkin lymphoma (iNHL) (hazard ratio 0.52; 95% confidence interval [CI] 0.39, 0.69; 1-sided p=0.000002; Matasar et al. Lancet Oncology 2021). The objective response rate and complete response (CR) rates were also significantly higher in the C+R arm (81% and 34%, respectively) vs the P+R arm (48% and 15%, respectively). Whereas R administration was limited (odd cycles 1 to 9), C was administered until progression or unacceptable toxicity. Patients who discontinued then entered active follow-up and had CT scans on a regular schedule. This included a number of patients with objective responses who stopped treatment prior to documented progression. To provide insight on the optimal treatment duration for patients achieving a best overall response of partial response (PR) or CR with C+R treatment, we compare the duration of response (DoR) in patients after stopping treatment vs DoR in those who remained on treatment.
Methods: CHRONOS-3 was a randomized, double-blind, placebo-controlled, Phase III study. Patients with iNHL (follicular lymphoma grades 1-3a, lymphoplasmacytic lymphoma/Waldenström macroglobulinemia, marginal zone lymphoma, small lymphocytic lymphoma) who relapsed after the last R-containing regimen and were progression-free and treatment-free for ≥12 months (mo) after the last R-containing regimen, or for >6 mo if unwilling/unfit to receive chemotherapy, were randomized 2:1 to receive C+R or P+R. C 60 mg flat dose was given via a 1-h i.v. infusion on days 1, 8, and 15 (28-day cycle) and continued until disease progression or unacceptable toxicity. R 375 mg/m 2 was given i.v. on days 1, 8, 15, and 22 during cycle 1 and on day 1 of cycles 3, 5, 7, and 9. Tumors were assessed by CT or MRI at screening and every 8 weeks during year 1, every 12 weeks during year 2, and every 24 weeks during year 3 and onwards. Response was assessed by blinded central review. DoR for patients with a PR or CR after stopping treatment was based on either the date of the last dose or the date of first response for patients whose scans occurred after stopping treatment.
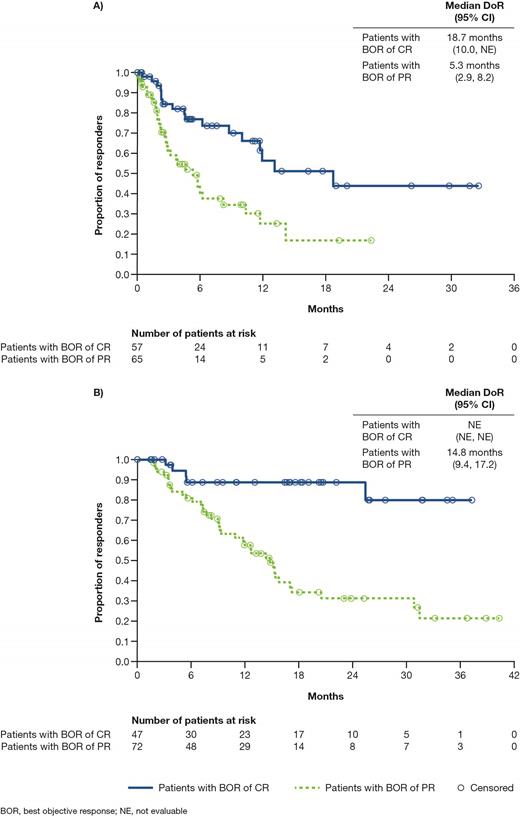
Results: As of August 31, 2020, 307 patients were randomized to C+R and 151 patients to P+R. In the C+R arm, 104 patients (34%) had a best overall response of CR, 137 patients (45%) had a PR, 5 patients (2%) had a minor response, and 2 patients (0.7%) had a very good partial response. Overall, median DoR was 20.4 mo (95% CI 17.0, 30.8) with a median time to first objective response of 1.8 mo and a median time to CR of 3.7 mo. Patients with a CR did not reach median DoR; 80% estimated response rate at 12 mo and 69% at 24 mo. Patients with a PR had a median DoR of 12.3 mo (95% CI 10.2, 15.3). A total of 57 patients with a CR and 65 patients with a PR stopped treatment before progression, with 71/122 (58%) patients discontinuing due to an adverse event. Median DoR before treatment discontinuation was 3.8 mo for patients with a CR and 1.7 mo for patients with a PR. Of patients with objective responses stopping treatment before progression, the total median DoR was 18.7 mo (95% CI 10.0, not evaluable) for those with a CR (n=57) and 5.3 mo (95% CI 2.9, 8.2) for those with a PR (n=65; Figure 1A). In contrast, of patients with objective responses who remained on treatment until progression or until the time of database lock, the median DoR was not reached for those with a CR (n=47; 89% estimated response rate at both 12 and 24 mo; Figure 1B) and the median DoR was 14.8 mo (95% CI 9.4, 17.2) for those with a PR (n=72; Figure 1B).
Conclusions: For patients with iNHL treated with C+R and achieving a CR, sustained responses were seen in some patients upon cessation of treatment before disease progression, although the DoR was longer for patients with a CR who remained on treatment. The DoR for those achieving a PR was markedly less upon cessation of treatment than for patients who remained on treatment. Together, these results support the trial design of treatment to progression, but further suggest that discontinuation of treatment for patients with relapsed iNHL treated with C+R may be an option for some patients achieving a CR. Analysis of factors associated with a sustained CR upon stopping treatment are ongoing.
Matasar: Pharmacyclics: Honoraria, Research Funding; Rocket Medical: Consultancy, Research Funding; Janssen: Honoraria, Research Funding; Teva: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Bayer: Consultancy, Honoraria, Research Funding; IGM Biosciences: Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Merck Sharp & Dohme: Current holder of individual stocks in a privately-held company; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Merck: Consultancy; TG Therapeutics: Consultancy, Honoraria; ImmunoVaccine Technologies: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Juno Therapeutics: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding. Özcan: AbbVie: Other: Travel support, Research Funding; Archigen Biotech: Research Funding; Bayer: Research Funding; Celgene: Research Funding; F. Hoffmann-La Roche Ltd.: Other: Travel support, Research Funding; Janssen: Other: Travel support, Research Funding; MSD: Research Funding; Takeda: Honoraria, Other: Travel support, Research Funding; Amgen: Honoraria, Other: Travel support; Bristol Myers Squibb: Other: Travel support; Abdi İbrahim: Other: Financial relationships; Jazz Pharmaceuticals: Other: Financial relationships; Sanofi: Other: Financial relationships. Jurczak: AbbVie, AstraZeneca, Bayer, BeiGene, Celtrion, Celgene, Debbiopharm, Epizyme, Incyte, Janssen, Loxo Oncology, Merck, Mei Pharma, Morphosys, Novo Nordisk, Roche, Sandoz, Takeda, TG Therapeutics, Pharmacyclics, Affirmed, Gilead Sciences, Nordic Nanovecto: Research Funding; AstraZeneca, BeiGene, Janssen, Loxo Oncology, Sandoz, Roche: Membership on an entity's Board of Directors or advisory committees; European Medicines Agency, Sandoz-Novartis, Janssen China R&D, BeiGene, Epizyme, Acerta, AstraZeneca: Consultancy; Maria Sklodowska-Curie National Research Institute of Oncology: Current Employment; Jagiellonian University: Ended employment in the past 24 months. Mongay Soler: Bayer HealthCare Pharmaceuticals Inc.: Current Employment. Cao: Bayer HealthCare Pharmaceuticals Inc.: Current Employment. Hiemeyer: Bayer AG: Current Employment. Childs: Bayer HealthCare Pharmaceuticals Inc.: Current Employment. Zinzani: ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmuneDesign: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp & Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal